Lighting? What does that have to do with color or perception? Well lots of course, otherwise I wouldn’t be writing about it  Light is how we see color in the first place – light bounces off objects and into our eye – and not all lights are made equal. Have you ever noticed the color of an object change when you walk out of a store and into daylight outside? The property of having different colors in different lights is called metamerism . . . but personally I think this is a rather flawed concept as ALL objects are metameric to some extent (in an extreme case, all objects are black when there is no light, or all objects are reddish when viewed in pure red light). Of greater interest than these extreme cases though are the cases we encounter every day.
Light is how we see color in the first place – light bounces off objects and into our eye – and not all lights are made equal. Have you ever noticed the color of an object change when you walk out of a store and into daylight outside? The property of having different colors in different lights is called metamerism . . . but personally I think this is a rather flawed concept as ALL objects are metameric to some extent (in an extreme case, all objects are black when there is no light, or all objects are reddish when viewed in pure red light). Of greater interest than these extreme cases though are the cases we encounter every day.
Most photographers know that outdoor photos have the most “natural” color rendering, while photos under incandescent lights (ie standard house lights) look yellow, and photos under fluorescent lights look greenish. Many photographers are forgetting this concept as cameras get better and better at auto white-balancing, but it’s an important thing to understand nonetheless. And of course, it’s the whole reason you have to (or should) white balance your photos. The reason photos look different under different lights is because the light sources have different spectra. Light is made up of waves of different wavelengths, and these wavelengths are perceived by your eye to be different colors. Back to the extreme case of a red light, all objects will look red because the light emits the red wavelength (around 700nm wavelength if you’re interested), and an object can only reflect or absorb red – its quite unlikely that the object will absorb red and emit a different color (it’s possible, but these are the exceptions to the rule).
You can probably start to see why it’s important to have a balanced light source – if your light doesn’t emit anything in the green wavelength range, then green objects have nothing to reflect and will look black. Your brain is really good at tricking your eyes though. So when we’re inside and the room is light by incandescent lighting we don’t stand around and wonder why everything looks so yellow – our brain figures out where the light is coming from, takes stock of the color of the light source, and uses that information to filter the colors of every other object we look at. A camera, however, doesn’t have that luxury (though it certainly tries hard with the auto whitebalance) . . . and we don’t have that luxury when we look at a photograph. The photo looks yellow because the scene WAS yellow, and our brain tricked us into believing that it wasn’t.
So what’s the big deal if our brain tricks us? Let’s take a look at some spectra of light sources. Here is a typical incandescent bulb plotted as relative intensity of light at the various wavelengths (mapped as colors here so it”s easy to look at):
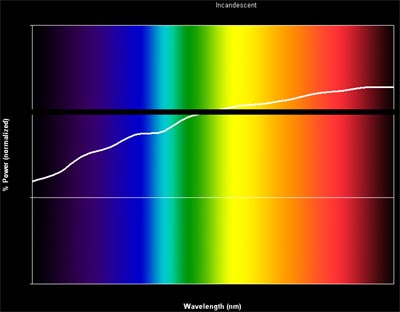
Not the black line running across the graph – that’s what a perfectly balanced spectrum would look like. So you can see the incandescent spectrum is severely lacking in the blue range, about right in the greens, a little high in the yellow, and fairly high in the reds. Your eye perceives blue and yellow to be opposites, and green and red to be opposites (more on that some other time, for now take my word on it). So the fact that incandescent lamps (and by the way, this includes quartz halogens as well!) have too little blue AND too much yellow compounds our perception of the scene being yellow. The bigger issue though is that blues and purples just plain don’t look right in incandescent lighting because they have very little incoming light to reflect (a huge problem for photography). And if your object has an optical brightener (which reflects in the UV by the way), it will look dingy under a tungsten bulb.
Ok, how about fluorescents? Fluorescents work differently than tungsten based bulbs – instead of heating up a metal which then emits energy from all of its electrons evenly, fluorescent bulbs stimulate a set of materials at specific energy levels which then fluoresce with a characteristic wavelength. There are lots of coatings and other modifications added to fluorescent bulbs, but here is what the spectrum typically looks like:
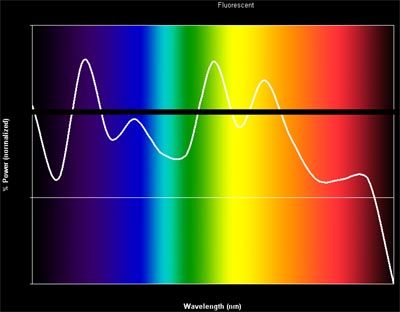
Woah! What’s going on there? Each of the peaks in this spectrum represents the characteristic fluorescent wavelength of one of the components of the bulb. And the valleys? These are spots where we don”t know of any economical materials that fluoresce at that wavelength. So it’s no wonder things look green under fluorescent light (note the large spike in the green wavelengths). But we can always white balance the photo to pull back the green. The more important issue is that there are many colors in the fluorescent spectrum that simply don”t exist (or exist at VERY low levels). So for example red objects and certain bluish-green objects will never look right in fluorescent light. And as before, the problem only gets worse in a photograph (remember, if the object didn’t have any light to reflect you won’t be able to recover or bump up that color in the photo – the color simply won’t exist!). The most devious part about the fluorescent spectrum is that it is often difficult to predict if a particular object will look ok or not – the missing wavelengths are very specific, so one bluish-green object might look fine, while one with a slightly different hue looks terrible!
Alright, so are there any solutions? Well, not really. At least not for photography. Stick with tungsten (incandescent) bulbs, and turn them up really bright (the advantage of using quartz halogen bulbs is that you can make them very bright) so that you at least get some blue light for the camera to pick up . . . and then white balance. Or shoot outside, that’s of course the best solution 
For daily use in your house there actually is a great solution, but a word of warning – don’t use these lamps for photography! GE has come out with a new type of incandescent bulb called the Reveal, which does an amazing job of eliminating some of the characteristic yellow cast of tungsten lights. And while your brain is quite good at tricking your eyes, it’s very impressive the difference these bulbs can make in your house – eliminating dingy yellows and brightening blues colors.
So why not use the GE Reveal lights for photography? Let’s take a look at the spectrum for this bulb:
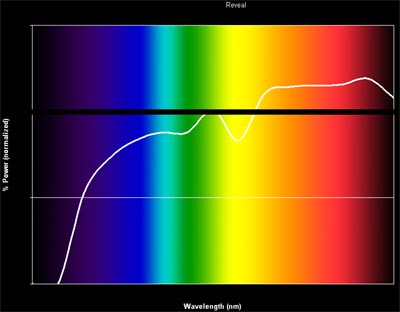
Ew, what happened there? GE added a material to the coating of this bulb which absorbs (primarily) yellow light. So there is less yellow in the resulting spectrum (and thus yellow objects look less yellow, and blue objects look more blue), but there is clearly not any more blue which as I mentioned earlier is the real problem with incandescent bulbs. In fact in a bit of a conundrum, it appears the GE Reveal might actually have LESS blue than a traditional incandescent! So it may work wonders on tricking your eye and brain, but it certainly won’t do any favors for your camera. In fact, because the spectrum is now non-uniform, you may actually have a more difficult time white balancing.
So there you have it. Buy GE Reveal light bulbs for your house. Don”t use them for photography 
















